Quick start to PIM & B2B eCommerce in the medical industry: Why MVP proved the winning formula

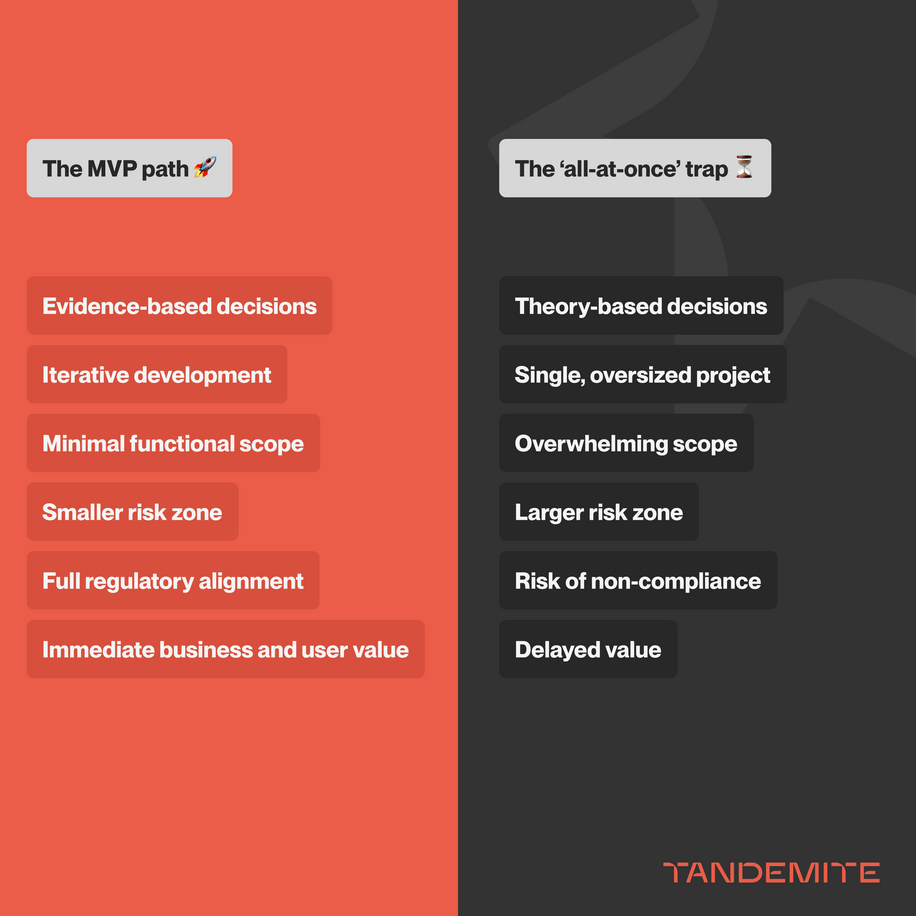
Comprehensive, all-at-once rollouts often sound like progress but tend to create delays and frustration. That’s why, with ZARYS, we chose a different approach: iterative development driven by MVP. Instead of an overwhelming scope, we focused on essentials, creating a platform that was easy to maintain and adaptable to change.
Medical supply for hospitals, wholesalers, and healthcare facilities represents a high-stakes domain: thousands of SKUs, multilingual usage documentation, diverse sales units, strict requirements for batch and expiry tracking, regulatory compliance, and contract-specific pricing. When tackled as a single, oversized project, the result is often decision paralysis, uncontrolled scope expansion, and reduced team engagement.
The ZARYS International Group implementation highlights a more strategic path. Product Information Management and B2B eCommerce platforms were developed in successive waves: beginning with a minimal, stable version and then expanding capabilities. This staged approach delivered tangible results – rapid PIM deployment, SAP integration the following month, and finally the launch of the eZARYS sales system.
In this article, you’ll discover how MVP helps you focus on what really counts, and how to keep your digital transformation moving after the first stage.
Why choose MVP over a full, „all-at-once” transformation?
I. Immediate business and user value
MVP allows organizations to deliver the first live features and orders within weeks instead of waiting quarters. This creates early momentum: sales team has live demonstrations for clients, marketing team builds campaigns on real use cases, and you collect meaningful feedback along with the first revenue streams. It also lowers the chance of drifting into a „never-ending project.”
II. Clearer, evidence-based decisions
When rules and data models live only on paper, hidden mistakes creep in. By validating them on real transactions, the team avoids theory traps. Concrete metrics – like how often a feature is used, how long tasks take, or how reliable the data is – make the right path obvious and keep the project on track.
III. Smaller risk zone
Starting with a limited number of product families and markets narrows the scope of possible issues. This way, you avoid the burden of fixing thousands of records or multiple integrations down the line. Instead, you fine-tune details early and scale safely in stages.
IV. Stronger, more resilient teams
With short sprints, a clear Definition of Done, and well-structured priorities, project fatigue is reduced. Incremental progress creates a sense of control and achievement, helping to sustain motivation and prevent burnout over the long term.
Speed matters in medtech – but so does compliance. That’s why an MVP must combine both: rapid value delivery and full regulatory alignment. What does this look like in real life? It’s the smallest possible set of complete data and workflows that let you list products, apply contract-based pricing, and process orders while fully aligning with MDR and local regulations.
It’s important to understand that a true MVP isn’t a cut-down version of the future system. It’s the first stable building block, strong enough to support every next step.
Minimal functional scope for MVP
„Does this scenario sound familiar?” Let’s look at the root causes:
- „Catalog still lives in Excel and emails” → Missing single source of truth and version control.
- Different IFU versions in circulation → No structured repository and no assignment rules per market/channel.
- Errors caused by wrong EAN codes → No validation of attributes and no mapping logic across packaging levels (unit/pack/carton).
- Prices or inventory not matching the contract → Disconnected integrations and no consistent publishing of price+stock+product.
- One attribute update stops publication → Workflow too rigid, with no split between critical and non-critical rules.
For anyone facing these challenges on a daily basis, the best response is MVP: an approach designed to create order in small, manageable phases and, in doing so, reduce the overall risk of the project.
„Our philosophy is to get the first version live quickly, by following the MVP path. We encourage clients not to lock themselves into projects that take two years to deliver. A project stretched out that long risks losing touch with the market, missing business opportunities, and draining people’s energy. It’s better to move fast, then improve step by step and add new features along the way.” — Maciej Pałubicki, CEO Tandemite
MVP PIM (Pimcore) – non-negotiable components
1) Data model
- Categories and product families (np. „Gloves”, „Syringes”).
- Critical attributes with their types, units, and dictionaries (e.g., sterile/non-sterile, latex/nitrile).
- Units and packaging conversions (1 carton = 10 packs = 500 units). The system must enforce consistency so that edits cascade across levels.
- Product variants (size, length, color).
The foundation of the MVP is a product data framework that anticipates medical requirements from the outset: UDI/DI identifiers, GTIN/EAN codes, product classifications, sterility status, sterilization method, storage parameters, expiration period, and packaging layers (unit, pack, carton, pallet), each carrying its own unique EAN. While completeness across the entire catalog is not mandatory from day one, the fields themselves must exist, with correct types and validations in place, and the workflow should require them before any product can progress to the „Ready for Sale” state.
2) Approval workflows / validation rules / data completeness and quality checks
- Every status requires its mandatory elements; without them, the process does not move forward. There are no shortcuts outside the workflow.
- A common path: Draft → Review → Legal/Regulatory → Ready for Sale → Published.
- Fields are required depending on status, channel, or language (e.g., an English product description must be present to sell in foreign markets).
- Duplicate prevention is part of the process (e.g., product name, description, or EAN cannot be repeated).
- For documents, a PIM MVP should include a repository with versioning, expiry tracking, and a matrix that maps documents to countries. IFUs, leaflets, datasheets, certificates, and manufacturer declarations all need to be up-to-date. If a required document is missing or expired, the workflow blocks publication. This minimum framework secures compliance with both global regulations and local language requirements.
The medical industry is driven by audits. To succeed, your MVP should already include a comprehensive audit trail (who made which changes and when), validation rules (EAN standards, numeric limits, minimum image quality, per-channel and per-language coverage), and status controls that block progression when critical data or documents are absent. This approach strengthens overall quality while simultaneously providing a clear record of due diligence during regulatory reviews.
3) Import–export and mapping (in phases)
- Large-scale imports often stall projects. A controlled approach (3–4 iterations of 5–10% of the portfolio) delivers far better results. Each iteration concludes with a retrospective, ensuring mapping corrections, deduplication, dictionary refinement, and stronger validation.
- A robust PIM system must allow for channel-specific completeness criteria (ERP, web shop, marketplace, distributor) and data transformations prior to publishing, such as shortened product texts or standardized images.
MVP B2B (Shopware): scope designed to accelerate revenue
Strategic goal: empower the logged-in business client to see their negotiated terms and place orders autonomously.
At its core, a B2B MVP should contain:
1) Customer login with custom price lists and tailored assortments, using visibility rules to target segments precisely.
2) Seamless merge of product content (PIM) and transaction data (SAP) at display time.
- Product data is the domain of a PIM system: structured images, descriptions, unit definitions with conversions, an EAN for each unit, and PDF attachments like leaflets, IFUs, or compliance documentation.
- Business data belongs to ERP: customer-specific pricing, contract terms, credit limits, reliable ATP levels, lead times, and allocation logic under constrained supply. The B2B platform acts as the presentation layer, showing the right information for the right logged-in account.
3) Streamlined checkout with cart functionality.
4) Fast and intuitive product search.
5) Customer accounts with a complete order and payment history.
For the medical world, the MVP scope should expand to include fast ordering (by pasting SKU/GTIN lists or importing CSV files), lists for repeat purchases, basic user roles and approval flows (buyer → approver), clear communication about units and packaging rounding, as well as consent and warning steps for sterile items.
Full substitute and supply chain modules are typically deferred to later waves. Yet adding a “recommended substitute” attribute and availability messages to the MVP provides immediate strategic value, enabling sales teams and customers to react fast and avoid time-consuming firefighting by phone.

How to measure whether your MVP really works?
Track these KPIs to see the real impact:
- What is the typical timeframe between kick-off and reaching the “Ready for Sale” milestone, or making the product available in the B2B sales channel?
- What percentage of SKUs comply with completeness rules across channels, languages, and product families?
- How many product entries are rejected at the integration stage, and whether the causes are regulatory or technical?
- How many duplicates appear each month, and how often are corrections needed?
- What portion of orders must be fixed because of issues like unit mismatches?
- Is B2B platform conversion rising, thanks to visible pricing and stock, effective search, and a smoother checkout?
- How often do customers report mismatched price/availability?
Would you like this approach to work for you?
„PIM and B2B implementation is about acting within months, not stretching it over years. Releasing a foundational version quickly and refining it iteratively is the way to go.” — Maciej Pałubicki, CEO, Tandemite
In medical B2B, “big bang” projects rarely survive the pressure of regulations, complex data, and stretched teams. MVP changes that; it delivers early revenue, operational savings, and full compliance with quality standards like validation, completeness, and approvals, all while laying out a clear roadmap for growth. Start with a portion of the catalog, manage contracts and limits, learn what drives results, and expand step by step. This is the fastest route to measurable value and a strong foundation – the perfect balance of agility and security that medical manufacturers and distributors are looking for. With PIM and B2B e-commerce up and running quickly, scaling to new languages, markets, and features is straightforward and low-risk.
Thousands of SKUs, multiple sales models, multilingual documentation, contract pricing, spreadsheets alongside ERP. It sounds like a challenge, but it’s also an opportunity. In this environment, MVP is the strategic advantage. Partner with us, and we’ll launch the essential components quickly. With the right foundations, your business can grow in a safe, structured, and future-proof way. Chaos is replaced with purpose: shared goals, clear responsibilities, fast decisions, and customer focus.





